Journal of Drug Delivery Science and Technology中科院3區,影響因子4.9
時間: 分類:SCI論文百科 次數:
今天介紹生物醫藥方向的3區期刊,Journal of Drug Delivery Science and Technology是中科院3區刊物,影響因子4.9,期刊旨在推動制藥和藥物遞送技術的創新發展,為臨床用藥提供科學依據,下面展開具體的介紹。
1、期刊基本信息
期刊名稱:Journal of Drug Delivery Science and Technology
創刊時間:2004
影響因子:4.9
實時影響因子:4.806
五年影響因子:5.2
中科院分區:3區
JCR分區:1區
自引率:8.2%
年發文量:1084
發行頻率:雙月刊
2、期刊基本指標分析
(1)影響因子:Journal of Drug Delivery Science and Technology影響因子近2年較為穩定,最新影響因子4.9,實時影響因子4.806,5年影響因子5.2。
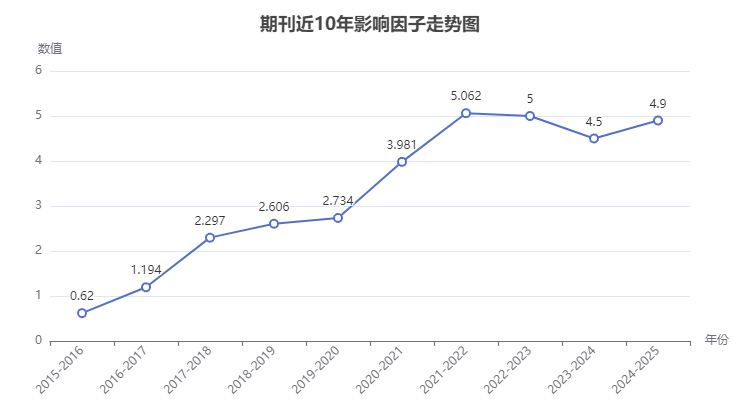
(2)分區:期刊位于中科院大類-醫學3區,在小類-藥學中位于3區,投稿難度一般。JCR數據顯示,期刊長期在Q1區,投稿有一定門檻。
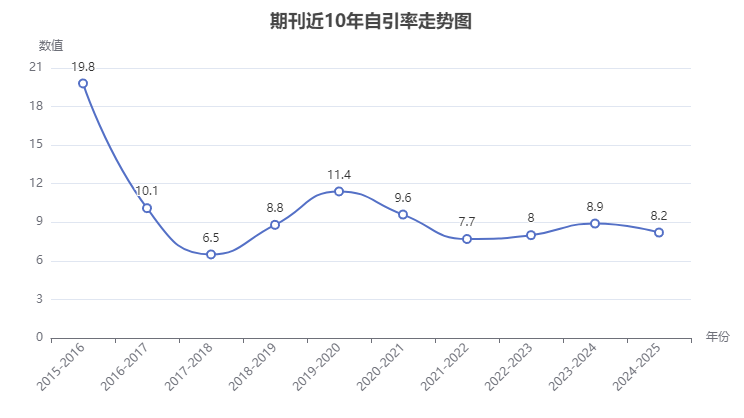
(3)自引率和引用關系:期刊自引率8.2%,沒有預警風險,還是比較安全的刊物。
3、期刊收稿范圍和稿件類型
收稿范圍:主要接收所有藥物劑型研究,包括控釋、生物利用度和藥物吸收、納米藥物、基因遞送、組織工程。
稿件類型:原始研究、綜述、短通訊。
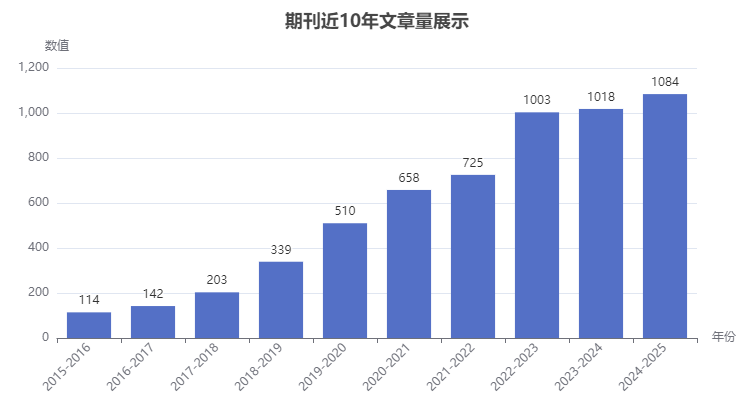
4、年發文量及國人占比
年發文量逐年上升,最近一年發文量在1084,預計可能進一步上升,錄用機會大了很多。從數據庫信息顯示:埃及知識庫、德黑蘭醫科大學和印度國家醫藥教育與研究所的發文量前三。近三年,中國地區的發文量第2,對國人友好。
5、審稿周期
Journal of Drug Delivery Science and Technology官網顯示,初審只需8天,審稿87天,提交到接受需要115天。近期出版的3篇文章,最快2個月錄用,最慢6個月。
具體時間如下:
第一篇:12月17日提交,6月1日接受。
第二篇:2月18日提交,5月28日接受。
第三篇:3月21日提交,5月31日接受。
6、投稿經驗分享
有4名學者分享了相關經驗,具體如下:
學者1:審稿比較慢,4個月的等待太漫長了。但結果是好的。
學者2:這個雜志處理稿件的效率實在很低,underview時間很長。
學者3:2個審稿專家,審稿周期近兩月,審稿意見共10余條,大多都不難回復。
學者4:審稿速度太慢,太浪費時間,審稿5個月卻拒稿了。
學者5:2個審稿人,審稿很快,2-3個周就有審稿意見,一次大修一次小修,小修后第二天就接受了,編輯很負責,很好得雜志。
7、期刊近期接收的文章如下
1)Bardoxolone methyl improves survival and reduces clinical measures of kidney injury in tumor-bearing mice treated with cisplatin
Acute kidney injury (AKI) occurs in approximately one-third of patients treated with cisplatin and there is an outstanding need for mitigation strategies to decrease the frequency and severity of cisplatin-ind...
Authors:Lauren E. Thompson, Stacey M. Tuey, Paola Garcia Gonzalez, Carly S. Chesterman, Courtney D. McGinnis, M. Scott Lucia, Lauren M. Aleksunes, Charles L. Edelstein and Melanie S. Joy
Citation:AAPS Open 2025 11:6
Content type:Research
Published on: 3 March 2025
2)Atezolizumab neutralizing assay development: a novel way of evaluating assay cutpoint after sample pretreatment
Atezolizumab, a biotherapeutic monoclonal antibody directed against PD-L1, has been shown to be efficacious in multiple oncology indications. In clinical trials, a subset of patients developed anti-atezolizuma...
Authors:Rachel Melendez, Jane Ruppel, Cecilia Leddy, Jenny Yang, Ann Brady, Yenny Webb-Vargas, Jochen Brumm, Lynn Kamen and Yuan Song
Citation:AAPS Open 2025 11:3
Content type:Research
Published on: 3 February 2025
3)A digital platform for automated analysis of 1H NMR data: prototype framework of digital reference standard
This study introduces an innovative approach to automatically analyzing 1H nuclear magnetic resonance (NMR) data, integrating a quantum mechanical spectral analysis (QMSA) to enhance efficiency over manual data a...
Authors:Sunil Babu Paudel, Joo-Won Nam, Gonzalo R. Malca Garcia, Ben Shapiro, Pekka Laatikainen and Yang Liu
Citation:AAPS Open 2025 11:2
Content type:Research
Published on: 20 January 2025
4)Advancements in regulatory agility, regional collaboration, and digital transformation: insights from the Asia Partnership Conference of Pharmaceutical Associations (APAC)
The Asia Partnership Conference of Pharmaceutical Associations (APAC) examines recent developments in regulatory practices across Asia, focusing on regulatory agility, regional collaboration, and digital trans...
Authors:Sannie Siaw Foong Chong, Stephanie Hui Min Ong, Siew Mei Long, Masaaki Kanno, Usanee Harnpramukkul, Kum Cheun Wong, Asawari Sathaye, Mamta Singh, Manish Paliwal, Huyen Do, Helene Sou and Richard Simon R. Binos
Citation:AAPS Open 2024 10:14
Content type:Research
Published on: 16 December 2024
5)Metallic nanoparticles in malaria treatment: advances in therapeutics, diagnostics, and future prospects
Malaria remains one of the causes of extreme mortality in southern Africa and Southeast Asia. Despite extreme efforts to control and eliminate malaria, the appearance of drug-resistant parasites and their spre...
Authors:Shweta Sinha, Rakesh Sehgal and Bikash Medhi
Citation:AAPS Open 2024 10:13
Content type:Review
Published on: 2 December 2024
6)Poloxamers anchored with TAT enhance blood–brain barrier penetration of carbamazepine for the treatment of epilepsy: an in vivo study
Carbamazepine is a pharmacological medication commonly prescribed to treat epilepsy. Dose adjustments, poor bioavailability, and prolonged side effects present significant challenges associated with its use. P...
Authors:Farnaz Sotoudegan, Mohsen Amini, Mohammad Sharifzadeh, Nasrin Samadi and Farzaneh Sotoudegan
Citation:AAPS Open 2024 10:12
Content type:Research
Published on: 4 November 2024
7)Pharmacokinetic interaction between single and multiple doses of darunavir, in combination with cobicistat or ritonavir, and single-dose dabigatran etexilate in healthy adults
Darunavir (DRV) is a P-glycoprotein (P-gp) inhibitor. Dabigatran etexilate, prodrug of the anticoagulant dabigatran, is a P-gp probe substrate. This study evaluated the effect of single and multiple doses of D...
Authors:Sandy Van Hemelryck, Erika Van Landuyt, Jay Ariyawansa, Martyn Palmer, Martine J. C. Kothe and Caroline Pollefliet
Citation:AAPS Open 2024 10:10
Content type:Research
Published on: 14 October 2024
8)Pre-analytical stability of ocrelizumab in serum after delayed centrifugation of whole blood
Authors:Jeongsup Shim, Montserrat Carrasco-Triguero and Saloumeh K. Fischer
Citation:AAPS Open 2024 10:11
Content type:Rapid communication
Published on: 16 September 2024
總結來看:Journal of Drug Delivery Science and Technology長期位于中科院3區,發展較好,但是審稿速度一般,大概3-4月左右,對于從事創新研究,如納米技術、新型制劑開發等前沿方向的學者,可以積極投稿。
- International Journal of Fuzzy Systems 計算機4區,IF3.6,5天給第1次審稿決
- 警惕Journal of Analysis and Testing化學1區TOP期刊,是ESCI期刊并不是SCI期刊
- Journal Of Energy Storage期刊官網查詢,中科院二區期刊,IF9.8
- Journal of Cereal Science是幾區,幾分期刊,投稿論文格式要求匯總
- Geomicrobiology Journal期刊投稿格式要求,投稿是word還是latex模板
- Journal Of Neurology官網好找嗎?2024IF分值變化大不大
- Egyptian Informatics Journal人工智能3區期刊,投稿經驗分享
- International Journal Of Nanomedicine2區高分神刊,接收率80%
- 《Journal Of Ethnopharmacology》幾乎不退稿,錄用率>50%
熱門文章閱讀排行榜
- 電力工程審計論文可投稿的的核心期刊
2025-07-19瀏覽量:843
- Ophthalmology and Therapy影響因子3.2,眼科學3區,初審決定時間3天
2025-07-18瀏覽量:420
- 環境與地球科學學科英文期刊推薦,附帶精選環境、地球文章
2025-07-18瀏覽量:531
- International Journal of Fuzzy Systems 計算機4區,IF3.6,5天給第1次審稿決
2025-07-18瀏覽量:563
- Neuroscience Letters 投稿交多少費用?審稿快不快?錄用率高嗎
2025-07-18瀏覽量:780
- 食品領域sci二三區國人發文量大的期刊推薦
2025-07-18瀏覽量:592
- 神經內科SCI論文升職稱有用嗎
2025-07-18瀏覽量:529
- 神經疾病領域SCI期刊Neurodegenerative Diseases,2.6分,4區好中期刊
2025-07-18瀏覽量:532
- 泌尿外科醫師論文適合發的sci推薦,1區到4區全覆蓋
2025-07-15瀏覽量:717
- 無機化學sci二區期刊推薦
2025-07-14瀏覽量:1090
中文核心期刊推薦
-

-
中國人口·資源與環境
級別:北大核心,JST,CSCD,CSSCI,WJCI
ISSN:1002-2104
刊期:進入查看
格式:咨詢顧問
-

-
高等工程教育研究雜志
級別:北大核心,CSSCI,AMI核心
ISSN:1001-4233
刊期:進入查看
格式:咨詢顧問
-

-
南京農業大學學報·社會科學版雜志
級別:北大核心,CSSCI,AMI核心,社科基金資助期刊,
ISSN:1671-7465
刊期:進入查看
格式:咨詢顧問
-

-
新疆師范大學學報·哲學社會科學版雜志
級別:北大核心,CSSCI,AMI核心,社科基金資助期刊,
ISSN:1005-9245
刊期:進入查看
格式:咨詢顧問
SCI核心期刊推薦
-

-
Scientific Reports
數據庫:SCI
ISSN:2045-2322
刊期:進入查看
格式:咨詢顧問
-

-
ACTA RADIOLOGICA
數據庫:SCI
ISSN:0284-1851
刊期:進入查看
格式:咨詢顧問
-

-
Materials Today Communications
數據庫:SCI
ISSN:2352-4928
刊期:進入查看
格式:咨詢顧問
-

-
APPLIED SURFACE SCIENCE
數據庫:SCI
ISSN:0169-4332
刊期:進入查看
格式:咨詢顧問
-

-
PLANT JOURNAL
數據庫:SCI
ISSN:0960-7412
刊期:進入查看
格式:咨詢顧問
-

-
SCIENCE OF THE TOTAL ENVIRONMENT
數據庫:SCI
ISSN:0048-9697
刊期:進入查看
格式:咨詢顧問
-

-
PLANT DISEASE
數據庫:SCI
ISSN:0191-2917
刊期:進入查看
格式:咨詢顧問
-

-
BMC BIOLOGY
數據庫:SCI
ISSN:1741-7007
刊期:進入查看
格式:咨詢顧問
-

-
JOURNAL OF WATER PROCESS ENGINEERING
數據庫:SCI
ISSN:2214-7144
刊期:進入查看
格式:咨詢顧問
-

-
Journal of Materials Research and Technology-JMR&T
數據庫:SCI
ISSN:2238-7854
刊期:進入查看
格式:咨詢顧問









